Synta Pharmaceuticals Corp. (NASDAQ: SNTA)is a biopharmaceutical company focusing on discovering, developing, and commercializing small molecule drugs to extend and enhance the lives of patients with severe medical conditions, including cancer and chronic inflammatory diseases.
The company has two drug candidates in clinical trials for treating multiple types of cancer and various drug candidates in the preclinical stage of development. Its oncology product portfolio includes Ganetespib, an Hsp90 inhibitor in Phase 2b/3 clinical trial for various types of cancers including non-small cell lung cancer. Also in development is elesclomol, a mitochondria-targeting agent in Phase II clinical trials for ovarian cancer and Phase 1 clinical trial for acute myeloid leukemia. STA-9584, a vascular disrupting agent is also in preclinical development for prostate cancer. After researching Synta, I feel this is a rare company where the sky is the limit if its potential is reached. For now, it may be a company whose stock will be good to trade based on several catalysts. Eventually, it is possible for Synta to be a huge revenue producer. Let's get into the reasons why.
I would like to start off with what the company refers to as its Galaxy trial. In September, Synta provided an update from an interim analysis of the Phase IIb portion of this trial. It is a Phase IIb/III study designed to evaluate the efficacy and safety of the company's lead Hsp90 inhibitor, Ganetespib, as second-line treatment for advanced non-small cell lung cancer (NSCLC). The results showed good tolerability for the combination of Ganetespib and docetaxel which was presented by the company at ASCO this year. In addition, there were meaningful improvements in overall survival in patients receiving this combination compared to those receiving docetaxel alone.
I found a recent presentation from the company very interesting with regards to the Galaxy trial. Synta spoke about the development of the Hsp90 inhibitors which is focused on in this study. Its drug, ganetepsib, is a second generation hsp90 inhibitor which is showing a significantly better safety profile compared to the first generation inhibitors. The first generation displays a lack of strength treating the cancer while being toxic in the liver. Meanwhile, ganetepsib has shown 10-100x more potency, a low molecular weight, and the absence of serious liver or common ocular toxicities.
Some characteristics of the upcoming study are as follows:
· Number of patients to be enrolled - 500
· Treatment population - Stage IIIB/IV non-small cell lung cancer, adenocarcinoma
· Primary endpoint - overall survival
The company expects to complete enrollment and begin the trial by the end of this year, and expects a mature data set in the first half of 2013. The timeline detailed in the above referenced presentation shows the final data from the Phase III trial in the first half of 2014.
Many people have been asking Safi Bahcall, the CEO, about accelerated approval possibilities and trying to speed up the process of getting this drug to the market. I was very impressed by his response where he firmly specified the goals in this trial. They are acquiring a very rich data set which can make the Phase III trial very complete and without any problems. The goal is the big picture and extending the survival of the patients. The best way to demonstrate this is in Phase III. The primary goal of Phase II is noted on one of the company's slides, "De-risking Phase III."
In general, I don't really like to write about cancer companies unless I really find something that separates them from the others. In this case, a comment from the CEO made me start to actually think this company could be reaching new heights in cancer research. Mr. Bahcall said one physician spoke to him noting they have something "incredibly valuable," and have a "huge responsibility" to get this drug to patients. It was very clear by the comments made that Synta truly believes they have something very exciting in the works.
Synta notes that the response rate is currently only 8-9% in patients that ganetepsib could hopefully serve once approved. When this happens, the expectation is for a much higher number. It is a highly unmet need. In addition, for the seven highly populated countries that could benefit from ganetepsib, 160,000 new patients need treatment each year.
A drug currently used for treatment in combination with chemotherapy for lung cancer is bevacizumab. This drug was initially approved in 2004 but has not been without issues. At one point bevacizumab was approved for breast cancer by the FDA, but the approval was revoked in November 2011. The approval was revoked because, although there was evidence that it slowed progression of metastatic breast cancer, there was no evidence that it extended life or improved quality of life. It also caused adverse effects including severe high blood pressure and hemorrhaging. In 2008, the FDA gave bevacizumab provisional approval for metastatic breast cancer, subject to further studies. In July 2010, after new studies failed to show a significant benefit, the FDA's advisory panel recommended against the indication for advanced breast cancer. The manufacturing company Genentech requested a hearing, which was granted in June 2011. The FDA ruled to withdraw the breast cancer indication in November 2011. Doctors may sometimes prescribe it for that indication, although insurance companies are less likely to pay for it. The drug remains approved for breast cancer use in other countries including Australia.
Another upcoming catalyst for Synta Pharmaceutical is the expected results from its Phase I compassionate use trial, where a single patient was treated with Elesclomol.
The study was designed as a dose-escalation of elesclomol sodium in a single patient with relapsed or refractory acute myeloid leukemia (AML). The primary objectives of the study are to characterize safety, tolerability, and pharmacokinetics.
Elesclomol is a first-in-class, investigational drug candidate that triggers programmed cell death (apoptosis), in cancer cells through a novel mechanism: disrupting cancer cell energy metabolism by selectively targeting the electron transport chain in cancer cell mitochondria.
Elesclomol initially was developed in a partnership with GlaxoSmithKline (NYSE: GSK). It was given fast track and orphan drug status from the FDA in 2008 for the treatment of metastatic melanoma. Synta Pharmaceuticals announced on February 26, 2009 the suspension of all clinical trials involving elesclomol due to safety concerns. In March 2010, Synta announced that the FDA had approved resuming its clinical development.
In a small, randomized Phase II study, elesclomol was shown to significantly increase progression-free survival in people with metastatic melanoma when given in addition to paclitaxel. Paclitaxel is a mitotic inhibitor used in chemotherapy developed by Bristol-Myers Squibb (NYSE: BMY).
One company looking for similar leukemia treatment is one I've written about in the past, Ariad Pharma (NASADAQ: ARIA). This company's stock price has soared over the last three years from about $0.45 to over $25 on speculation that its lead drug candidate, Ponatinib will be very successful.
Ponatinib, a BCR-ABL inhibitor, is designed for heavily pretreated patients with resistant or refractory chronic myeloid leukemia (CML), or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
While the chemical make-up of elesclomol is different than Ariad's Ponatinib, the end goal remains common which is to help people with CML. While Elesclomol is in the early stage of testing here, any company that has a potential treatment for CML and other various cancers tends to garner a good deal of speculation value.
Another catalyst event for the company is the preliminary results from the Phase II CHIARA trial, expected by the end of this year. The trial is evaluating Ganetespib monotherapy for the treatment of ALK (anaplastic lymphoma kinase) + NSCLC patients not previously treated with a direct ALK inhibitor such as crizotinib.
The CHIARA (CHaperone Inhibition in Alk Rearranged lung cAncer) trial is a single arm, Phase II study evaluating Ganetespib monotherapy in patients with Stage IIIB/IV non-small-cell lung cancer harboring an ALK gene rearrangement and who have not been previously treated with a direct ALK inhibitor. The primary endpoint of the study is objective response rate.
Finally, data from Phase II proof-of-concept trial of single-agent Ganetespib in HER2+ or triple negative breast cancer patients is expected in by the end of this year as well.
Some insiders appear to like the prospects of their company, as they have been buying lately:
| Dec 3 04:50pm | Nov 29 2012 | SNTA | Synta Pharmaceuticals Corp | Singh Amar | Sr. VP, Chief Business Officer | 3,969 | Option payment at $8.21 | $32,585 |
| Nov 14 06:48pm | MNov 12 2012 | SNTA | Synta Pharmaceuticals Corp | Kovner Bruce | Director, 10% Owner | 37,700 | Purchase at $6.91 | $260,457 |
| Nov 14 06:45pm | MNov 12 2012 | SNTA | Synta Pharmaceuticals Corp | Kovner Bruce | Director, 10% Owner | 37,700 | Purchase at $6.91 | $260,457 |
| Nov 13 09:06am | Nov 12 2012 | SNTA | Synta Pharmaceuticals Corp | Bahcall Safi R | President, CEO | 10,000 | Purchase at $7.12 | $71,200 |
The stock has a $18.25 price target from the Point and Figure chart, and six analyst buy ratings, one neutral rating and 0 sell ratings with an average target price of $10.80.
As insiders and traders seem to be positioning themselves for a nice entry point lately, short sellers seem to be sleeping at the wheel with Synta:
| Share Statistics | |
| Avg Vol (3 month): | 497,048 |
| Avg Vol (10 day): | 331,900 |
| Shares Outstanding: | 61.82M |
| Float: | 36.89M |
| % Held by Insiders: | 42.89% |
| % Held by Institutions: | 32.90% |
| Shares Short (as of Nov 15, 2012): | 7.25M |
| Short Ratio (as of Nov 15, 2012): | 17.10 |
| Short % of Float (as of Nov 15, 2012): | 19.60% |
| Shares Short (prior month): | 7.09M |
Because the stock has a relatively low float, a surge of buying volume is likely to cause a nice short squeeze here.
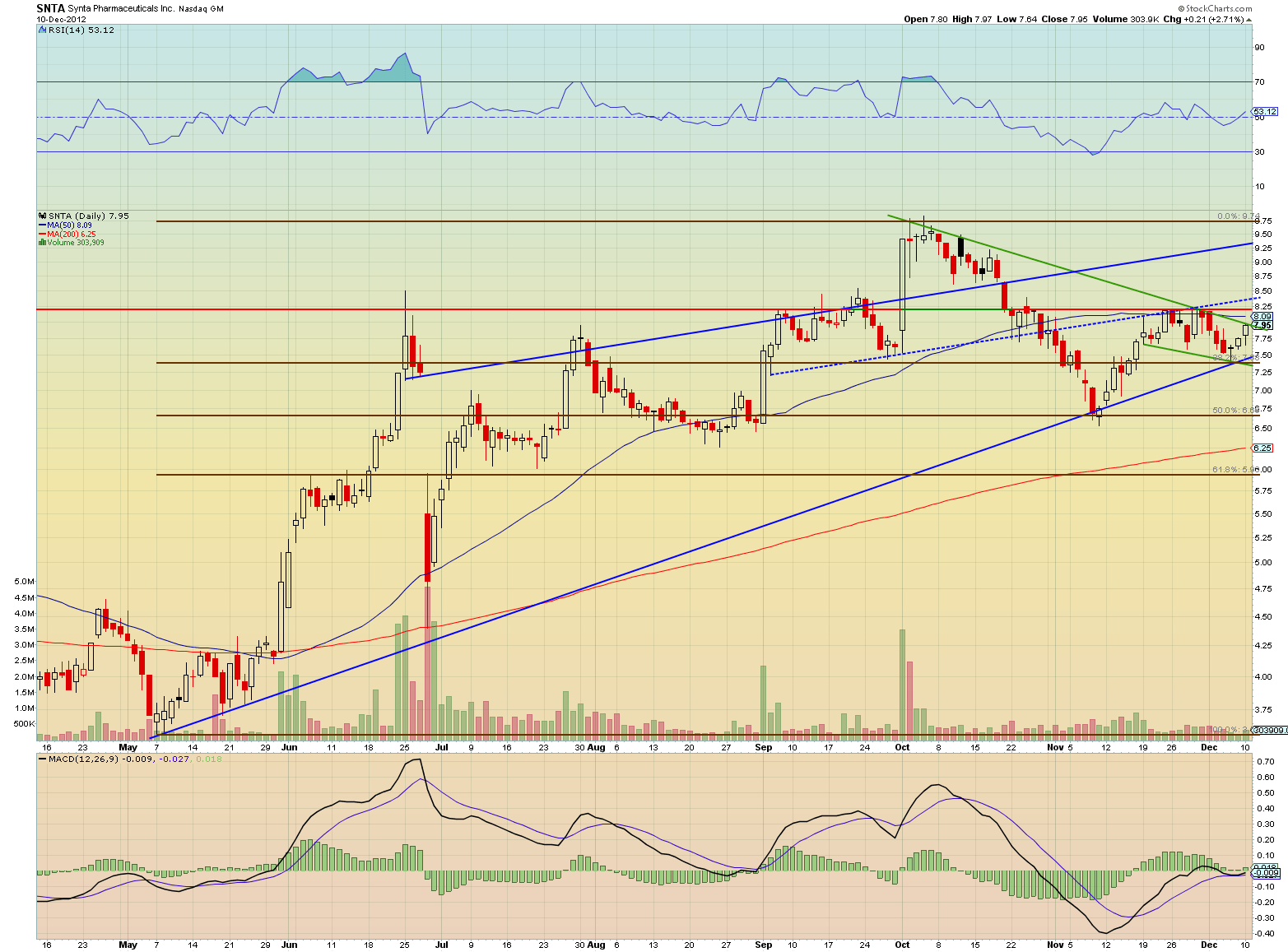 The chart above shows a positive MACD convergence and strong trendline support. The stock is now wedged in a tight ranged pennant. Any significant buying volume will break out the stock as high as $9.50, retesting the 52 week highs.
The chart above shows a positive MACD convergence and strong trendline support. The stock is now wedged in a tight ranged pennant. Any significant buying volume will break out the stock as high as $9.50, retesting the 52 week highs.
My 4 to 6 week Price target opinion is $9.50 - $10 My longer term target is uncertain as we need to see how all these programs goals turn out. If positive, then a price over $30 in 5 years is possible.
Disclosure: I am long SNTA.
