EnteroMedics Inc. (ETRM)
I bought a nice amount of shares. Catalyst trade run up target opinion: $.4.40. Positive data release: Gap to $7. 1 year: $15 (my opinions only)
According to company press releases, conference calls, and my correspondence with investor relations, Phase III data from its Recharge study on its flagship product, The Maestro System, is due to be released around mid Q1. In 2009, traders bet the stock to near $30 a share on anticipation of the company's "EMPOWER" study succeeding. But, after it ultimately failed to meet both its primary and secondary endpoints, the stock tanked to below $10 a share, and became somewhat forgotten by the market.
After the failure of the EMPOWER study, the company stated;
Based on the analysis to date, the control arm of the trial, which was intended to be inactive, apparently provided a low-intensity blocking signal that introduced VBLOC Therapy in human subjects.
After the addressing the issues and meeting with the FDA, the company made some modifications to the system, and designed a new Phase III trial.
"The ReCharge Study," requires the company show a 10% greater Excess Weight Loss (EWL) from randomization with the Maestro System after 12 months of VBLOC therapy compared to control by BMI method, and to observe clinically meaningful responder rates in the treatment arm of 20% and 25% EWL from implant at 12 months (not statistically based).
In 2010, the company's Australian cohort showed results that surpasses the new trial's requirements. In the Australian cohort, a total of 83 subjects were enrolled at two centers, with 61 subjects implanted. Main outcome measures were morbidity, mortality, and excess weight loss at 12 months. Results include;
- Mean 12-month EWL was 25% for the treatment group and 17% for the control group;
- Weight loss was linearly related to hours of device use; subjects with greater than or equal to 9 hours/day use achieved 37% and 21% mean EWL (treated versus control, p = .02); and
- No therapy-related serious adverse events or deaths were reported across the entire study population.
Considering all the factors here, I expect EnteroMedics to announce positive data sometime around the middle of February.
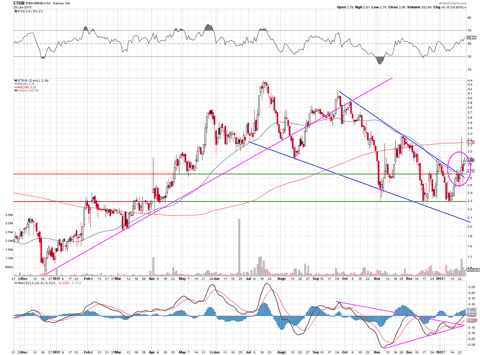
The chart above indicates to me that the stock has found two bases, one at $2.30, another at $2.65, and has recently broke its mid-term down trend line. With this consolidation above $2.72, I expect much more upside in the short term, with $3.30 as being the first level before making a second move to $4.40 or higher.
Morbid obesity is an ever growing problem worldwide, and having the option of a device like the Maestro System as a choice over gastric bypass surgery and potentially dangerous pills could really make a difference in the lives of those who might need it. It could also mean big dollars for the company, which in turn would mean huge gains for the company's stock holders. Both traders and investors should keep a close eye out on this one as I feel it has strong potential to be a multi-bagger this year.
Disclaimer: This article/post is intended for informational and entertainment use only, and should not be construed as professional investment advice. They are my opinions only. Trading stocks is risky -- always be sure to know and understand your risk tolerance. You can incur substantial financial losses in any trade or investment. Always do your own due diligence before buying and selling any stock, and/or consult with a licensed financial adviser. StockMatusow.com or any of its affiliates/subsidaries are not licensed brokers.

I just picked up a couple thousand shares. Sold my Kerx for a nice profit