Danny has explained Affimed's science below, which for some may be a bit complex. Tomorrow, I will look to break down the technical explanations in more simpler terms -- this is a big reason many up and coming TRUE potential biotechs get 'no love.' The Average investor and even more so, institutional funds (hedge, mutual, and otherwise) simply do not understand a lot of this science. Biotech made a huge run up in a 'pile on' scenario where fund managers were following everyone else, having very little clue as to what they were buying. Even your big name tutes with their newer guys brought on since 2009, only know an 'easy money' market so when biotech was sold off, many of these 'brilliant' people had no idea what they were selling as they had no idea what they were buying in the first place.
We want to make sure you fully understand our picks and our reasoning. So, this one Danny covers the technical science, and I will break it down in simpler terms tomorrow in a write up and audio review.
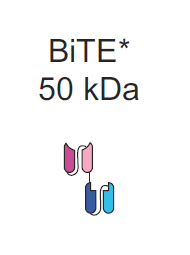
Bispecific antibodies (BsAbs) are a rapidly emerging area of immuno-oncology, following the approval of Amgen’s Blincyto (Blinatumomab) for the treatment of relapsed/refractory B-cell ALL. Blincyto, based originally on Micromet's Bispecific T-cell Engager (BiTE) platform, is designed to engage CD-3 on T-cells and direct them to B-Cells by binding to the surface antigen, CD-19 In many ways, BsAbs function as a molecular bridge linking immune cells to a number of disease targets. They represent a compelling platform alternative to Car-T based therapies proposed by Juno Therapeutics and Kite Pharma.
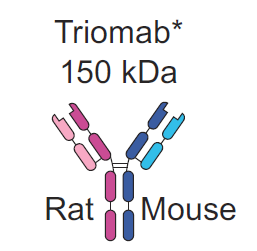
However, not all bispecifics are created equal. The key to maximizing efficacy and minimizing off-target effects with antibodies lies within molecular design. Bispecifcs can be roughly divided into two subclasses of design: those that include an Fc region, which functions as a stabilizing trunk - and those without such a region. Including an Fc region is a bit of a contested issue within the molecular biology community. On the plus side, Fc domains add stability which translates directly to a longer half life in the blood. In the case of Fresenius's Catumaxomab, based upon the Triomab platform, the antibody benefits from a serum half life of approximately 2.5 days. To the contrary, Fc domain bearing antibodies suffer from a significant molecular weight, which adversely impacts tumor penetration. In addition the Fc domain is a nonspecific binding site, which means that immune effector cells such as NK and macrophages end up competing with neutrophils at the antibody site. Neutrophil binding thus lowers the immune response in the body and reduces efficacy.
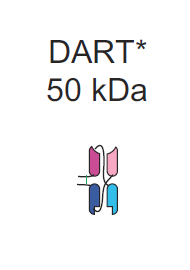
Of the bispecifc formats which do not bear an Fc domain, the most clinically advanced are Amgen's BiTEs, Affimed's Tandem Diabodies (TandAbs), and Macrogenics's dual affinity retargeting molecules (DARTs). Since Blincyto has the broadest safety and efficacy profile as well as FDA approval, we will use this as a benchmark for our analysis. In a clinical trial, Blincyto showed remarkable efficacy in relapsed ALL. Considering BiTEs have an approximate molecular weight of 50-55 kDa, one third of the typical Fc-bearing format, one can conclude that smaller antibody size leads to improve penetration. There are limitations to this approach of miniaturization in BsAbs. 50kDa is below the lower threshold for glomerular filtration, meaning that the antibody is filtered from the blood by the kidneys. As a result, Blincyto is administered via continuous IV, due to its ~2hr half life in the blood.
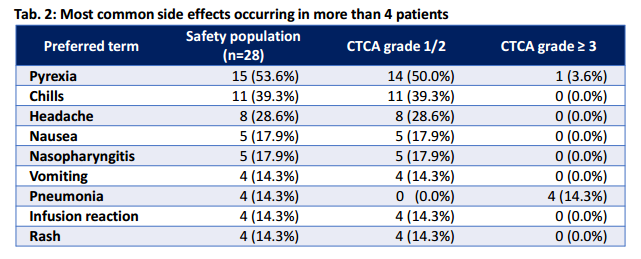
This small particle size also allows the drug to cross the blood brain barrier, as demonstrated by Blincyto's neural toxicity in clinical trials. CNS toxicity was observed in 15-20% treated with Blincyto including seizures, cerebellar symptoms, and encephalopathy or confusion. Of these adverse events, all were reversible via discontinuation of treatment. Another common issue with T-cell engagement is Cytokine Release Syndrome (CRS), characterized by fevers, chills and hypotension. This too was observed in clinical trials, see table below (Source: Ribera J-M, Ferrer A, Ribera J, Genescà E. Profile of blinatumomab and its potential in the treatment of relapsed/refractory acute lymphoblastic leukemia. Onco Targets Ther. 2015;8:1567–1574.)
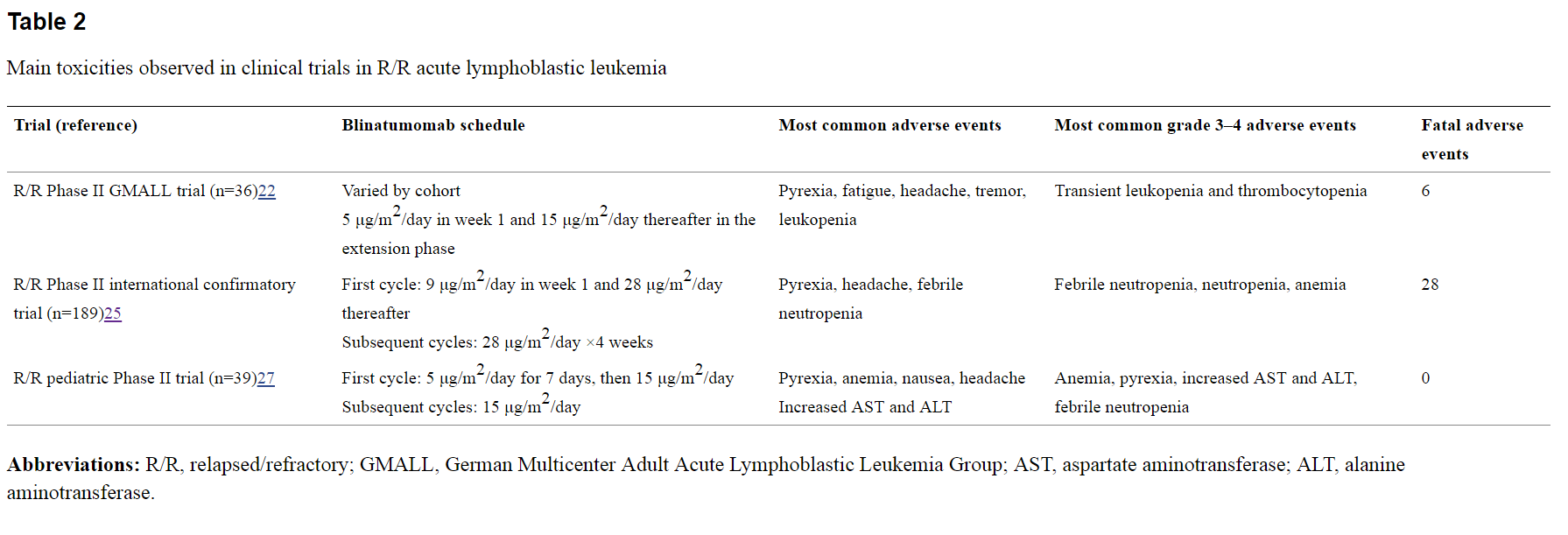 Doctors can manage CRS symptoms with pre-treatment using steroids like Dexamethasone, but such routes carry their own risks.
Doctors can manage CRS symptoms with pre-treatment using steroids like Dexamethasone, but such routes carry their own risks.
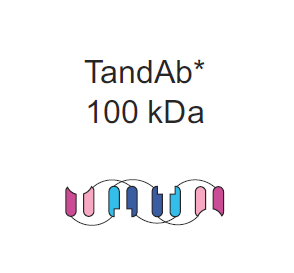
As observed, there are a few meaningful ways to improve the safety and efficacy profile of BsAbs that lack Fc domains. One, It is evident that there is a limit to molecular size, namely that these should be above the glomerular cutoff of approximately 50-70 kDa. Two, more binding sites can leader to higher specificity at the tumor site and prevent off target binding. Three improvements in stability of BsAbs without the need to include an Fc domain would dramatically improve quality of treatment. Bearing this in mind, TandAbs should in theory show superiority to alternatives such as BiTEs and DARTs. As demonstrated below we can see a few significant structural differentiators between the three competitors. For reference I also included the Fc-bearing Triomab used in Catumaxomab.
As demonstrated, TandAbs are clearly above the minimum size needed to avoid kidney filtration, eliminating the need for continuous IV. Its cross-linked structure provides increased stability without having to make the sacrifices associated by having an Fc domain. Both of these properties contribute to the antibody's 19 hour half life in the blood. It would appear that Tandabs rest in that key middle ground in terms of molecular weight; small enough to penetrate a tumor but not so small that toxicity becomes a significant issue. In addition TandAbs are unique among their BsAb peers in that they are tetravalent, meaning they have four specific binding sites. This creates added flexibility for the platform in tailoring design as well as increased specificity and avidity for the targets both on effector and target cell. Further it is possible to expand this flexibility to trispecific antibodies, which can in theory further improve the safety and efficacy profile by targeting combinations of surface proteins unique to the tumor site.
Though early, this hypothesis of improved safety and efficacy is supported by the phase one trial in Affimed's lead candidate AFM-13, a CD30/CD16a BsAb, in Hodgkin’s Lymphoma. Here we see a tolerable safety profile is presented. In this trial, the maximum tolerable dose was not reached and most side effects were grade 1/2 (mild to moderate) (source:http://www.affimed.com/pdf/affimed_afm13_dec14-ash.pdf)
AFM-13 is also unique in that it is the most advanced NK-cell engager in the clinic today. By replacing the Fc domain with CD16a (aka. FcγRIIIa), higher specificity to NK-cells and macrophages is achieved. This binding site is unique to these two effectors cells which means they will not have to compete with other white blood cells to attack the tumor.
AFM-13 has demonstrated even improved efficacy in a preclinical model with PD-1 checkpoint inhibition, which is being further investigated in a Phase 1 combo trial with Merck's Keytruda. (source: http://www.affimed.com/pdf/160330_afmd4q15_presentation_final.pdf)
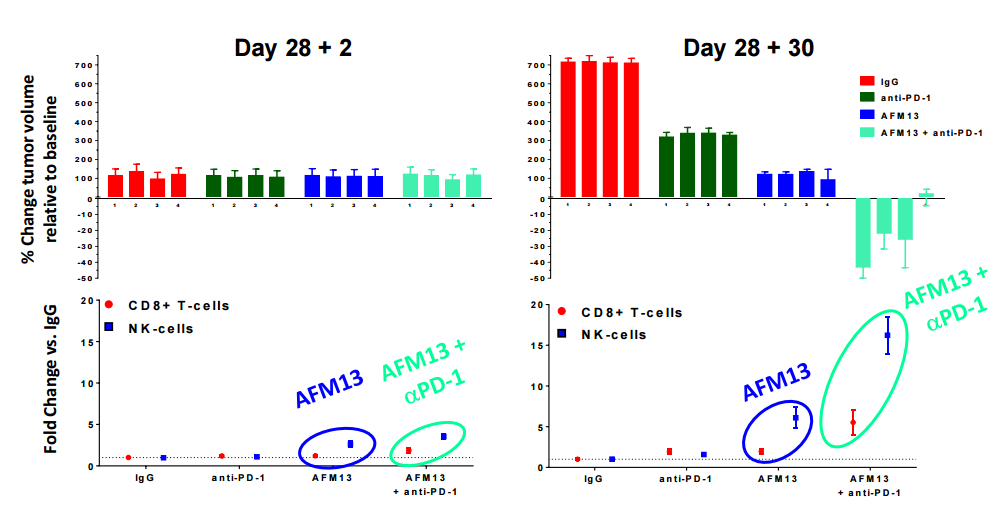
Affimed's preclinical programs hold a significant amount of promise as well. Of particular interest is their EGFRvIII targeting antibodies AFM-21 and AFM-22. EGFR is a key target for solid tumors, expressed with GBM, Colon, Head and Neck, Prostate, and Breast cancers. However the un-mutated EGFR expression is not just limited to cancer cells, meaning that generalized EGFR antibodies demonstrate off-target effects. 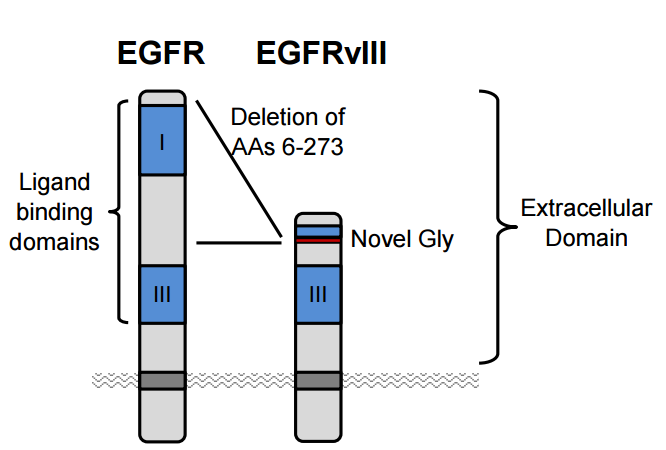
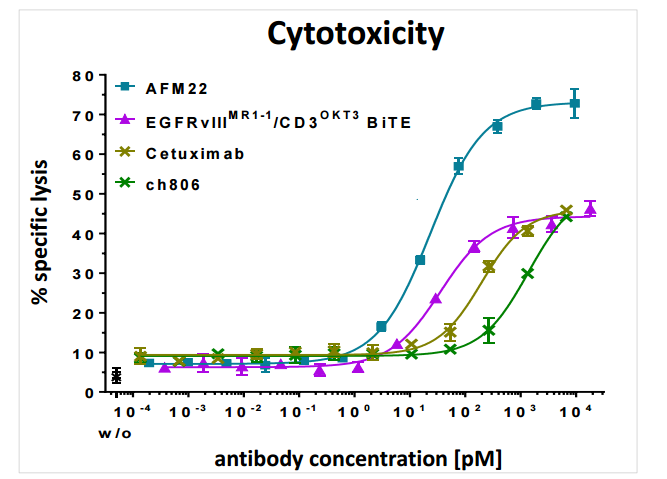
The most common mutation to EGFR, EGFRvIII is unique to tumor sites making it an attractive target for antibody therapy. Affimed's AFM21/22 represent the first bispecific antibody aimed at this mutation and in preclinical models shows superior efficacy to its monoclonal (single binding domain) antibody counterpart centuximab, which only targets EGFR. AFM-22 also showed superior cytotoxicity compared to a BiTE with the same binding domains. Despite BiTE's smaller size, AFM-22 demonstrates superior efficacy, likely attributed to its tetravalent structure. The increased avidity from possessing four total binding sites seems to be more than making up for size limitations at the site.
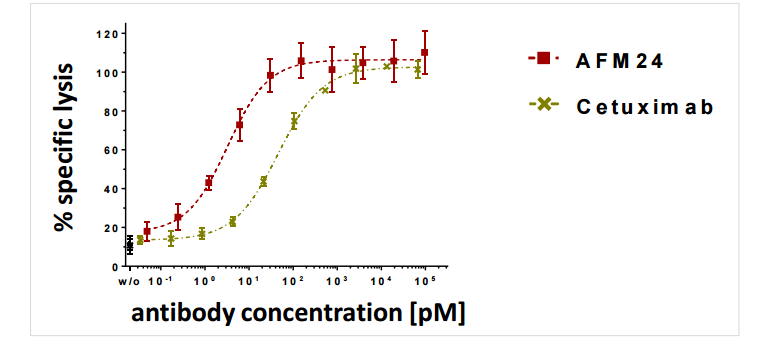
Currently, Affimed is reviewing preclinical data in depth to find the best antibody suited to this target, they are evaluating a CD3 binding antibody (AFM-21) and a CD16a binding antibody (AFM-22). In addition Affimed is testing a general EFGR antibody (EGFRwt) known as AFM24, which also demonstrates improvements in targeted cell death over Cetuximab. It should be noted that Cetuximab, approved in colon cancers was one of the best selling cancer drugs bringing in over 1 billion in peak sales in 2013.
Affimed's TandAb platform represents a unique oppertunity within the rapidly emerging immunotherapy field. While bispefics are generating increasing attention as an improved risk reward alternative over Car-T therapies, it is important to be discerning when selecting molecular design. As clinical development continues, we feel that Affimed will continue to validate the hypothesis of improved affinity, avdidity, and specificity directly translates to clinical benefit.