Trading biophama stocks before a catalyst event, if done correctly, can be a very profitable proposition. Some traders and investors who enjoy bringing more risk into play often times will hold through the actual catalyst event. While this does bring more risk into play, the reward from engaging in this can be a massive one. Today, I list a few small-cap companies and their respective catalyst events, along with some commentary on why I feel they might be worthy long term speculation investments. Investing for the long term in these small-cap biopharmas can be very risky because they can experience very volatile price moves, so it's wise to take this into consideration before making an investment in them.
ArQule Inc. (ARQL)
ArQule engages in the research and development of cancer therapeutics directed toward molecular targets and biological processes. Its lead product candidate tivantinib (ARQ 197) is an inhibitor of the c-Met receptor tyrosine kinase, which is in clinical trials for the treatment of liver cancer and colorectal cancer (CRC).
Phase II top-line data covering CRC is due out by the end of this year/early next year for ARQ 197. The data release will estimate the difference in progression-free survival between the study and control arms in subjects with CRC with wild-type KRAS who have received front-line therapy.
The company also plans to start a Phase III HCC trial soon for the drug, to be carried out under a Special Protocol Assessment from the FDA.
ArQule is also developing an Eg5 Inhibitor, a BRAF Inhibitor, an FGFR Inhibitor, and an AKT Inhibitor. So, the company has a deep pipeline along with the near term catalyst event of top line Phase II data, making the company's $178.30M market cap undervalued for a mid-stage Oncology biotech in my opinion. It also makes for a good catalyst trade opportunity.
| Net Institutional Purchases - Prior Qtr to Latest Qtr | |||
| Shares | |||
| Net Shares Purchased | 5,640,490 | ||
| % Change in Institutional Shares Held | 10.48% | ||
Institutions have increased their stake by over 10% in the company lately, which is usually a good sign.
Also of note, Edison Investment Research values ArQule at $5.40 a share, which equates to a market cap of about $376M, more than double the company's current market cap.
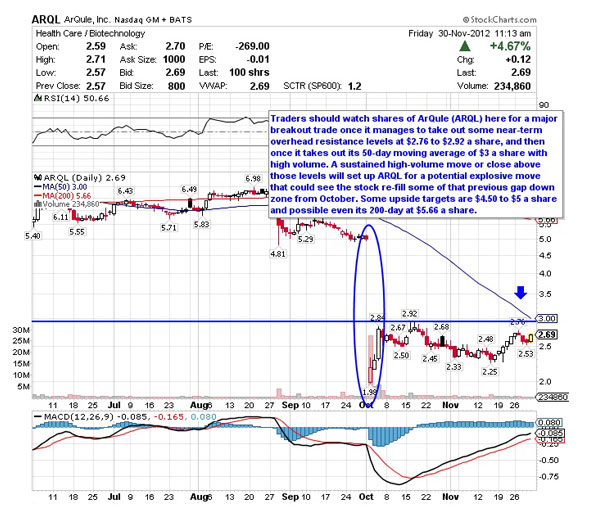
The above chart was taken from Robert Pedone's Street article published a couple of weeks ago. I spoke to Robert yesterday to get his updated take on the charts, and we both agree that if the stock breaks past $3 on decent volume, we could see an upwards gap fill to $4 rather quickly. With the data release expected any day now, I feel ArQule is a good long side trade for the short term. With good data, possibly a longer term buy and hold would be rewarded.
My price target opinion is $4, with a move over $4.50 if heavy volume materializes.
Sarepta Therapeutics (SRPT)
Sarepta focuses on the discovery and development of RNA-based therapeutics for the treatment of serious and life-threatening rare and infectious diseases. Its lead clinical candidate is Eteplirsen, which is in Phase II for the treatment of Duchenne muscular dystrophy (DMD), a rare form of muscular dystrophy that only affects young boys.
Sarepta announced on December 13th that it priced an underwritten public offering of 4,950,495 shares at a price to the public of $25.25 per share. While many believe dilution is counterproductive to a higher stock price, I would point out that Synta Pharma (SNTA) recently priced a secondary offering at a market price of $8.61 a share. Synta closed yesterday's session at $9.47, reaching my price target from an article I mentioned it in last week. Synta still has some legs left in its current run to reach $10 a share, since the company has four potential catalysts coming up. Therefore, Synta still remains on my list of catalyst trades and speculative investments for this week.
In early October, Sarpeta presented positive 48 week data on Epeplirsen, causing the stock price to skyrocket from $15 up to $45. The 60 week data is due in early January, and could have an equally huge impact on the stock price.
Sarepta has great potential in my opinion. With a possible treatment/cure for DMD, Sarepta could easily become a 5 bagger from its current price of $24.45. I consider the recent offering a good opportunity for those who enjoy engaging in a higher risk investment. If the company can ultimately prove Eteplirsen works, then the reward will be a massive one. The 60 week data release will be very important for both the company and its stock holders. If positive, expect the stock price to balloon much higher.
Acadia (ACAD)
Acadia focuses on small molecule drugs that address unmet medical needs in neurological and related central nervous system disorders. It has a pipeline of product candidates led by Pimavanserin, which is in Phase III development as a potential treatment for Parkinson's disease psychosis.
On December 12th, Acadia announced a private placement equity financing pursuant to which the company will receive gross proceeds of $86.4 million from the sale of its securities. Shares of Acadia's common stock will be sold at $4.43 per share, the closing market price on December 11, 2012. The private placement is expected to close on December 17, 2012 and is subject to the satisfaction of customary closing conditions.
The anticipated proceeds from the private placement will be used primarily to support completion of ACADIAs Phase III pimavanserin program, including its planned confirmatory Phase III pivotal trial in Parkinsons disease psychosis.
Acadia's stock has had quite a ride lately, gapping up over 200% on November 27th, reaching a pre-market high of $7.25. The huge gap up was caused by its drug Pimavanserin meeting the Phase III primary endpoint of reducing psychotic symptoms. Initially, in the pre-market session on the morning of the news, I shorted the stock for a quick and profitable scalp trade. This was not because I thought the company does not have massive potential, but because the gap was too large and the stock needed to consolidate at its new higher levels. Since the company has announced the financing, the stock has settled in nicely and I now feel it's worth a speculative investment.
Because Parkinson's disease psychosis has no current treatments, it qualifies as a drug designed to treat an unmet need. In the original Phase III study, Acadia had a failure in achieving its original primary endpoint for Pimavanserin. At the time, I wrote that the company might have designed the study incorrectly, and had a good chance to see success in a better thought out study.
The potential market is huge for Pimavanserin, if approved by the FDA, and I think it has a very good shot if the company continues on its current course. Also, since the company now has plenty of money, Acadia makes for a more stable speculative investment in my opinion. Keep a close eye on the news feed for Acadia if you decide to make an investment/trade in it. Additional positive news can rally the stock very hard, but negative news here can tank it equally as hard. It's my opinion that Acadia potentially offers a very large reward for those willing to take the risk.
Antares Pharma (ATRS)
Yesterday, Antares announced the submission of a New Drug Application (NDA) to FDA for OTREXUP (formely known as VIBEX MTX), a subcutaneous self injector designed to treat rheumatoid arthritis (RA), poly-articular-course juvenile RA, and moderate to severe psoriasis.
Used in an estimated 70% of patients alone and in combination with biological therapies, methotrexate (MTX) is a foundational disease-modifying anti-rheumatic drug for RA. Generally initiated orally at lower doses and titrated up, published studies have reported as many as 30% to 60% of patients experience gastrointestinal side effects with oral MTX. This can prevent further dose escalation or require discontinuation in some patients, which can be avoided by subcutaneous administration.
Paul K. Wotton Ph.D., President and Chief Executive Officer remarked:
The NDA submission of OTREXUP represents yet another significant accomplishment in the company's history. It is the first product designed for convenient subcutaneous delivery of methotrexate in patients with rheumatoid arthritis or psoriasis. We believe OTREXUP will benefit most patients that have not reached a satisfactory response to oral methotrexate alone or in combination with a biologic or another disease-modifying anti-rheumatic drug.
As I predicted, Antares did in fact file earlier than expected for OTREXUP. However, the stock price was somewhat flat yesterday. This could be in part due to the market not yet digesting this news, which equates to Antares potentially bringing OTREXUP to market faster than originally anticipated.
I also feel in part, that the company just does not tell its story to the retail market in a way that would be beneficial to itself and its shareholders.
I held Antares stock for 15 months before deciding to sell, mainly because I felt the long term on it would be "very long," and because I felt the company was not relating properly to the retail market as mentioned above. However, Antares is one of the safer small cap long term investments around in my opinion, if one is willing to wait five years or so for some big returns.
Antares is in a good cash position now, sitting on $70M or so. Also, Antares recently announced a 50/50 partnership with Teva (TEVA) for another one of its proprietary injectors, VIBEX 2. Antares is not a typical biotech company as the company does not engage in developmental new drugs. What Antares does is take existing drugs and make them more accessible via subcutaneous injections, many of them "needle free." Investors might also want to do the due diligence on the company's deep patent profile and future biosimilar products.
Antares should appreciate nicely over time as the company transitions into a full-fledged pharmaceutical company. I also believe it is possible Antares could be acquired by a larger pharma in the future because of its patent profile, so investors should consider this as well.
1 year price target opinion: $7, 3 years: $12, 5 years: 30+.
Hemispherx Biopharma (HEB)
Hemispherx engages in the clinical development of new drug therapies based on natural immune system enhancing technologies for the treatment of viral and immune based chronic disorders. Its products include Ampligen, an experimental drug under clinical development for the treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
This Thursday, the company has a scheduled Advisory Committee Members Meeting (ADCOMM) to discuss and decided whether or not to recommend for approval Ampligen, which is designed to treat Chronic Fatigue Syndrome (CFS).
I'm going to go against the majority thinking on this one and predict that the ADCOMM will vote to recommend Ampligen for FDA approval.
Currently, there are no approved treatments for CFS and patient advocacy groups are pushing hard to have one available that is both a safe and effective treatment.
The situation here reminds me a bit of when Arena (ARNA) had its weight loss drug Belviq receive a positive recommendation from its ADCOMM. Like with Ampligen, many argued that Belviq really did not show great efficacy in its clinical testing. Most well-known biotech writers dimissed Belviq giving it little chance for approval. However, I predicted it would receive a positive nod because there was great political pressure to get a weight loss drug out on the market. My review of Ampligen's data suggests the drug does treat CFS on many levels. While it might not be a great treatment for CFS, it would be the only treatment for it, if approved.
Given the current political atmosphere surrounding FDA ADCOMM's lately, I estimate a vote breaking 70-30 for recommending Ampligen for FDA approval.
However, just because it's my opinion Ampligen will get a positive recommendation, it does not mean it will which makes Hemispherx a very risky investment/trade. My price target opinion on a positive ADCOMM is $3, and $0.20 on a negative one.

Great article Scott!
I hope I have enough time to get rid of fold when it pops, to pick up more shares of
Thank you for all your assistance
Regards Jamy 🙂
Scott–what are your thoughts/read on FOLD after the news yesterday regarding PH III results? The placebo results seem kind of strange given that most expected that there would be little (if any) improvement in the placebo arm. My read is that the improvement of those receiving the drug would have been considered good had it not been for the “good” results in the placebo arm Trying to decide whether I should just dump this (for 40-50% loss) and move on to the next opportunity or buy on this dip and wait for the 12 month results. Keep up the good work–I’ve used your articles as a starting point for my own DD and been very happy with results to date!
Honestly, If it was me, I would not sell if I was thinking more long term. The Company seems bullish on it still, 12 month data coming and a Phase II on At2200. There could have been an issue with the controls in the study. We saw something like this with ACAD and they redid their Phase III, and got a positive result — look what happened to the stock there 🙂