Catalyst: XenoPort conducted an End-of-Phase 2 meeting with the FDA in which it received feedback that a proposed development program for XP21279, a novel prodrug of levodopa, could support a potential New Drug Application (NDA) submission under Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act. Based on its discussions with the FDA, XenoPort believes that a single, pivotal, Phase 3 clinical trial comparing optimized doses of XP21279 to Sinemet, along with an open-label safety study, could form the basis for an NDA submission as a potential treatment of the symptoms of advanced idiopathic Parkinson's disease. XenoPort plans to initiate certain activities in preparation for potential Phase 3 development of XP21279.
| Balance Sheet | |
| Total Cash (mrq): | 112.86M |
| Total Cash Per Share (mrq): | 2.63 |
| Total Debt (mrq): | 0.00 |
| Total Debt/Equity (mrq): | N/A |
| Current Ratio (mrq): | 8.93 |
| Book Value Per Share (mrq): | 2.18 |
| Shares Short (as of Nov 15, 2012)3: | 1.85M |
| Short Ratio (as of Nov 15, 2012)3: | 4.60 |
| Short % of Float (as of Nov 15, 2012)3: | 8.70% |
| Shares Short (prior month)3: | 1.70M |
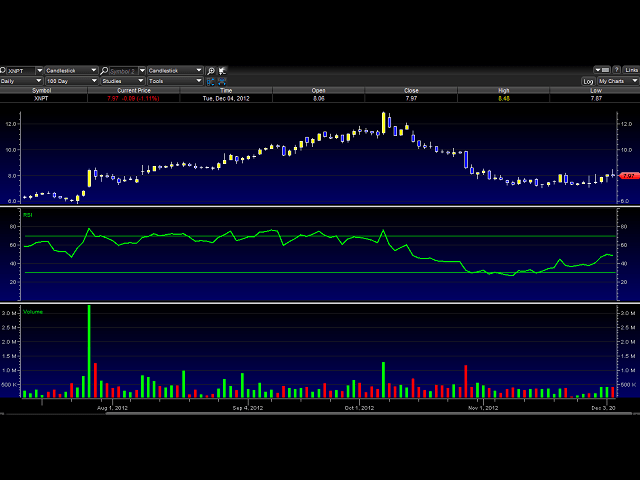
The chart is showing the very beginning of the " run - up" trade before the late December catalyst mentioned above. We can see the original trend line resuming after the mid term down swing. Skipping a full Phase III and going right to an FDA NDA filing would be huge for XNPT here. Saves a lot of money and means drug can potentially hit the market a lot faster. XNPT has plenty of money on hand, and with a market cap of $341.97M, it seems to me that it's undervalued by about $150M
Price target opinion by December 31st 2012 = $9